HPTN 083-01 is a clinical trial examining whether injectable cabotegravir (CAB) for PrEP (pre-exposure prophylaxis) is safe and acceptable for adolescent males [assigned male at birth –including transgender women (TGW), and gender nonconforming people]. This study will be enrolling about 50 participants at sites in Boston, Chicago, Denver, and Memphis.
What will happen during HPTN 083-01?
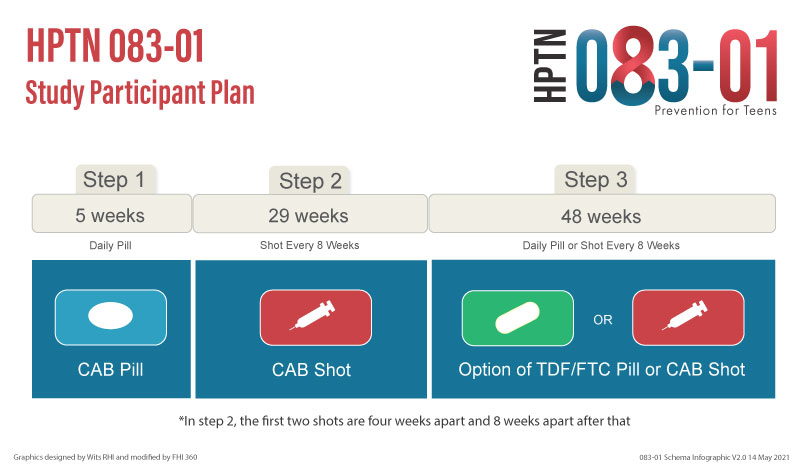
Participants will move through the study in 3 steps:
- Step 1: Participants will take one CAB pill every day for five weeks
- Step 2: Participants will receive a total of 5 CAB injections over 6 months
- Step 3: Participants will come to the clinic for study visits quarterly and have the option to take Truvada for PrEP for about one year or continue on CAB LA injections
.